Types and sources of radiation
Radiation is the release of energy in the form of moving waves or streams of particles. It has always been present and is all around us in many forms. There are 2 basic types of radiation: non-ionizing and ionizing.
Non-ionizing radiation
Non-ionizing radiation is relatively low-level energy that does not carry enough energy to remove electrons from atoms.
The following common applications use non-ionizing radiation:
- microwave ovens
- cell phones
- GPS devices
- television stations
- AM and FM radio
- baby monitors
- garage-door openers
Other forms of non-ionizing radiation are defined as extremely low frequency (ELF) waves. Sources include:
- the earth’s magnetic field
- magnetic field exposure (from being close to transmission lines)
- household wiring
- electrical appliances

Ionizing radiation
Some types of radiation have enough energy to knock electrons out of their orbits around atoms. This upsets the electron/proton balance and gives the atom a positive charge.
Electrically charged molecules and atoms are called ions. The radiation that can produce ions is called ionizing radiation.
There are many types of ionizing radiation, including alpha, beta, gamma, X-ray and neutron.
Alpha particles are made up of 2 protons and 2 neutrons. They have no electrons and carry a positive charge. Alpha particles are barely able to penetrate skin because of their size and charge, and can be stopped or blocked completely by a sheet of paper.
Beta particles are fast-moving electrons or positrons ejected from the nucleus of an atom. They are charged particles, about 1/7000 the size of an alpha particle. Because beta particles are smaller than alpha particles, they are more penetrating. However, beta particles can still be stopped by a small amount of shielding, such as a sheet of plastic.
Gamma radiation is a very penetrating type of radiation. It is usually emitted immediately after the release of an alpha or beta particle from the nucleus of an atom. This type of radiation can pass through the human body because it has no mass or charge. However, it is absorbed by denser materials, such as concrete or lead.
X-rays are a form of radiation similar to gamma radiation. They are produced mainly by artificial means rather than from naturally occurring radioactive materials. Like gamma radiation, they can be absorbed by denser materials.
Neutron radiation is created when neutrons are ejected from the nucleus by nuclear fission and other processes. The nuclear chain reaction is an example of nuclear fission. When a neutron is ejected from a fissioned atom, it causes another atom to undergo fission, and more neutrons are ejected.
The most effective shielding is provided by materials with a large amount of hydrogen atoms such as paraffin wax, plastic or water.
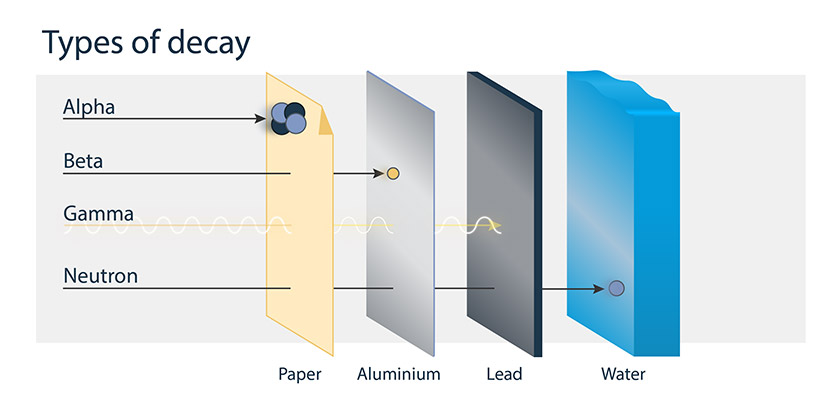
Text version
This image shows 4 types of radioactive decay and different types of shielding material that would block or reduce each type. Alpha radiation can be blocked by paper, beta radiation can be stopped by aluminum, gamma radiation can be reduced or absorbed by lead and neutron radiation can be absorbed by water.
Sources of ionizing radiation
People are constantly exposed to small amounts of ionizing radiation from the environment as they carry out their normal daily activities. This is known as natural background radiation. Radiation exposure also occurs as a result of medical procedures or treatments involving radioactive material.
Natural background radiation
Radiation has always been present and is all around us. Life has evolved in a world containing ionizing radiation, and our bodies have adapted to it.
The following section outlines sources of natural background radiation. Visit the Radiation doses page and fact sheet on natural background radiation for information on dose levels from these sources.

Text version
This image shows a family fishing and examples of various sources of natural background radiation from air, plants, cosmic rays, rocks and soil, water, food, and from our own bodies.
The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) identifies 4 major sources of public exposure to natural background radiation:
- cosmic radiation (space)
- terrestrial radiation (the ground)
- inhalation
- ingestion
Exposure from cosmic radiation
The earth’s outer atmosphere is continually bombarded by cosmic radiation. This type of radiation has fast-moving particles that exist in space. They come from a variety of sources, including the sun and other celestial objects in the universe.
Cosmic radiation is mostly made up of protons but can be other particles or wave energy. Some ionizing radiation penetrates the earth’s atmosphere and is absorbed by humans, which results in natural radiation exposure.
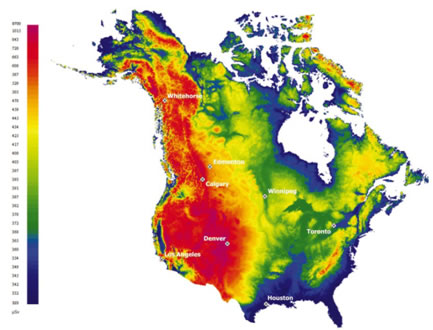
Doses due to natural sources of radiation vary depending on location and habits. Regions at higher altitudes receive more cosmic radiation than those at lower elevation.
The following map shows how levels of cosmic radiation vary with elevations above sea level, and longitude and latitude in North America. It also shows the annual outdoor effective dose in microsieverts (1 mSv = 1000 microsieverts) from cosmic radiation in North America.
Exposure from terrestrial radiation
Elements within the earth’s crust are a major source of natural radiation. The main elements are natural deposits of uranium, potassium and thorium. When these elements decay naturally, they release small amounts of ionizing radiation.
Uranium and thorium are found nearly everywhere. Traces of these minerals are also found in building materials, so exposure to natural radiation can happen indoors as well as outdoors.
Exposure through inhalation
Most exposure to natural radiation is caused by inhaling radioactive gases (for example, radon and thoron) that are produced by radioactive minerals found in soil and bedrock.
Radon is the largest source of natural radiation exposure on average. It is an odourless and colourless radioactive gas produced primarily by the decay of uranium-238. Radon is an inert gas, meaning that it does not react with the matter around it. It can readily move up through the ground and into the atmosphere.
Levels of radon and other radioactive gases vary greatly by location, depending on what the soil and bedrock are made of. These gases normally dilute to harmless levels in the atmosphere when they are released into the air. Sometimes they become trapped and accumulate inside buildings where they are inhaled.
Radon gas can pose a health risk to uranium miners, and several safety measures are in place in Canadian uranium mines to ensure worker safety. Radon can also be harmful to homeowners if it accumulates in the home. More information about radon gas and ways to control it can be found on the Health Canada website.
Exposure through ingestion

Naturally occurring radioactive isotopes (radioisotopes), such as potassium-40 and carbon-14, are found in food and drinking water and continually expose us to radiation. For example, vegetables are typically cultivated in soil and groundwater that contains radioactive minerals, so eating them results in internal exposure to natural radiation. Brazil nuts also naturally contain radium-226.
Naturally occurring radioactive isotopes have the same chemical and biological properties as their respective non-radioactive isotopes. Both radioactive and non-radioactive elements are used in building and maintaining our bodies.
The following table identifies the amount of radioactivity from potassium-40 contained in about 500 grams of different food products. A becquerel (Bq) is a unit of radioactivity, equal to 1 transformation (decay) per second.
| Food | Becquerels (Bq) per 500 g |
|---|---|
| Red meat | 56 |
| Carrot | 63 |
| White potato | 63 |
| Banana | 65 |
| Lima bean | 86 |
| Brazil nut | 103 |
| Source: Handbook of Radiation Measurement and Protection, Brodsky, A., CRC Press, 1978. | |
The human body contains several radioactive isotopes. The following table lists some isotopes naturally found in the body.
| Isotope | Amount of radioactivity in Bq |
|---|---|
| Uranium | 2.3 table 2 note 1 table 2 note 2 table 2 note 3 |
| Thorium | 0.21 table 2 note 2 |
| Potassium-40 | 4,000 table 2 note 2 |
| Radium-226 | 1.1 table 2 note 2 |
| Carbon-14 | 3,700 table 2 note 2 |
| Tritium | 23 table 2 note 4 |
| Polonium-210 | 40 table 2 note 2 table 2 note 3 |
Table 2 Notes
|
|
Artificial sources of radiation
There are 4 major sources of artificial radiation:
- atmospheric testing
- medical uses of nuclear substances
- industrial activities that use nuclear substances
- nuclear fuel cycle
Atmospheric testing
The atmospheric testing of atomic weapons from the end of World War II until as late as 1980 released radioactive material, called fallout, into the air. The fallout was absorbed by the environment as it settled into the ground.
Much of the fallout had a short half-life and has transformed to a stable element, but some radioisotopes continue to decay. People and the environment receive smaller and smaller doses from this fallout every year.
Medical uses of nuclear substances
Radiation has many medical uses. The most well known use is in X-ray machines, which use radiation to find broken bones and to diagnose diseases. In Canada, X-ray machines are regulated by Health Canada and provincial authorities.
Another example is nuclear medicine, which uses radioactive isotopes to diagnose and treat diseases such as cancer. In Canada, the uses of radiation in nuclear medicine, along with the related equipment, are regulated by the Canadian Nuclear Safety Commission (CNSC). The CNSC also licenses reactors and particle accelerators that produce isotopes used for medical and industrial applications.

Industrial activities that use nuclear substances
Radiation has many industrial uses. These range from nuclear gauges used to build roads to density gauges that measure the flow of material through pipes in factories.
Radiation is also used in smoke detectors and some glow-in-the dark exit signs, and to estimate reserves in oil fields. Sterilization also uses large, heavily shielded irradiators.
In Canada, all of these uses are licensed by the CNSC.

Nuclear fuel cycle
Facilities and activities involved in the nuclear fuel cycle can release low levels of radioactivity that contribute to the dose received by the public. In Canada, the entire nuclear fuel cycle, along with resulting dose levels, are regulated and enforced by the CNSC.
Nuclear power plants, for example, use fuel rods that contain uranium to drive a chain reaction. This produces steam and in turn drives turbines to produce electricity. Uranium mines, fuel fabrication plants, and radioactive waste facilities also release some radioactivity.

This image shows examples from various stages of the nuclear fuel cycle that can result in low radiation releases:
- uranium mining
- yellowcake (a concentrated form of uranium)
- fuel rods
- a nuclear power plant
Striking a balance
There is little we can do to change or reduce ionizing radiation that comes from natural sources like the sun, soil or rocks. This kind of exposure is never entirely free of risk, but is generally quite low. Sometimes, natural sources of radioactivity – such as excessive radon gas accumulation – may cause unacceptably high radiation exposure and need to be reduced with specific measures.
Ionizing radiation that comes from artificial sources and activities is controlled more carefully by striking a balance between these 2 factors:
- the benefits that the radiation provides to individuals and society
- the risks that the radiation imposes on the environment and all people in Canada
There has to be a net benefit to warrant the use of radiation. Radioactive isotopes, for example, are allowed in smoke detectors because smoke detectors save lives.
Dose limits for licensed activities in Canada are set and enforced to minimize radiation exposure, for both workers and all people in Canada. Uses of radiation are licensed by the CNSC. Licensees are required to keep all radiation doses as low as reasonably achievable (ALARA), taking social and economic factors into account.
Related links
Page details
- Date modified: