Atoms – Nuclides, isotopes and radioactivity
Everything around us is made up of atoms. A nuclide is a type of atom, and nuclides that are radioactive are called radionuclides. Radionuclides of the same element are called radioisotopes.
On this page
Atoms
An atom has protons and neutrons that make up the nucleus. Electrons orbit the nucleus in clouds (or shells) and carry a negative charge. The negative electrons are attracted to the positive nucleus by an electrical force. This attraction is what keeps the electrons orbiting the nucleus.
Protons are positively charged but neutrons don’t carry a charge. The nucleus therefore carries a positive charge.
The strong nuclear force binds the protons and neutrons to hold the nucleus together, despite protons repelling each other.
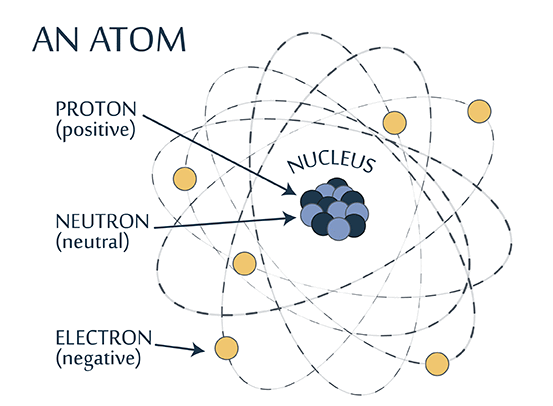
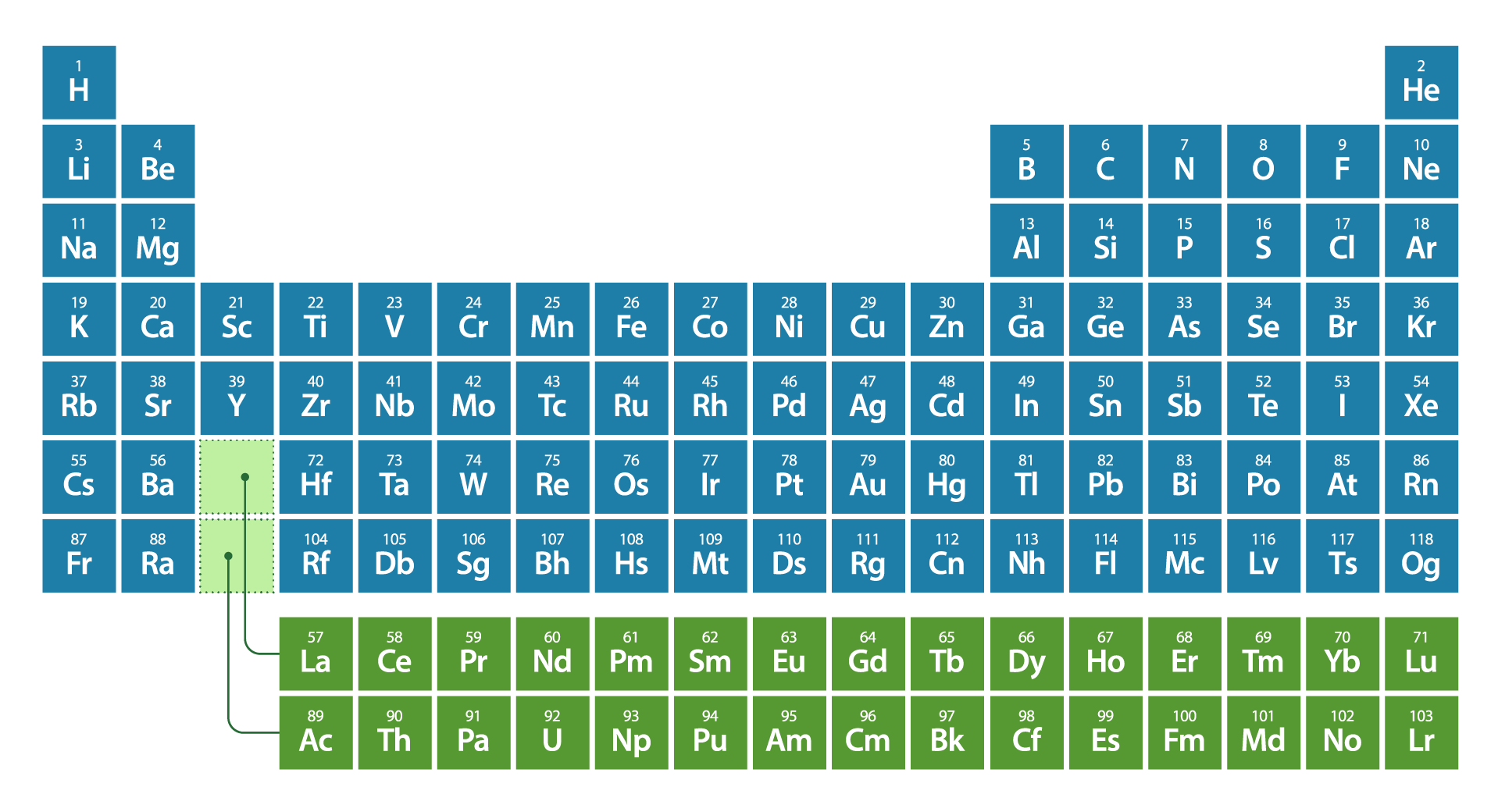
Each element is identified using a unique atomic number, which is also the number of protons in the nucleus of each atom forming that element. For example, carbon has the atomic number 6 on the periodic table because there are 6 protons in the nucleus of a carbon atom.
Periodic table of elements
An atom is stable when the number of neutrons and protons in the nucleus is balanced (although balance does not always mean an equal number of neutrons and protons). When there is a major imbalance between the number of neutrons and protons in the nucleus, an atom becomes unstable.
The atom may then transform by releasing energy to become stable. This process emits energy in the form of ionizing radiation, and is called radioactive decay.
Atoms from 1 or more elements can combine to form a larger compound called a molecule. For example, a molecule of water (H2O), is made up of 2 atoms of hydrogen (H) combined with 1 atom of oxygen (O).
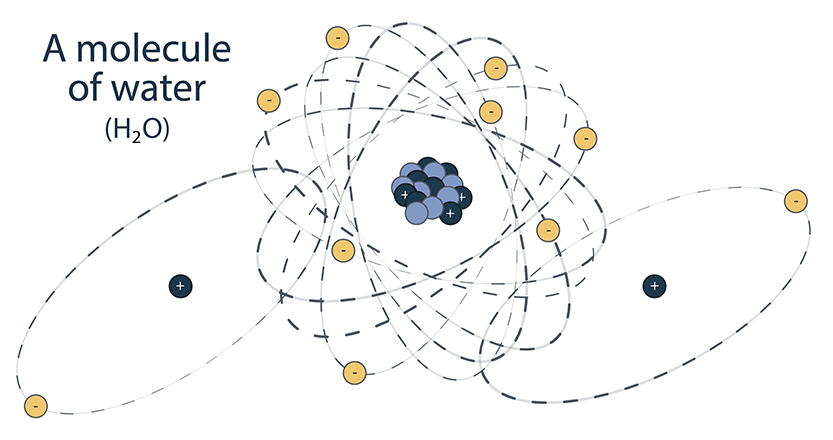
Nuclides
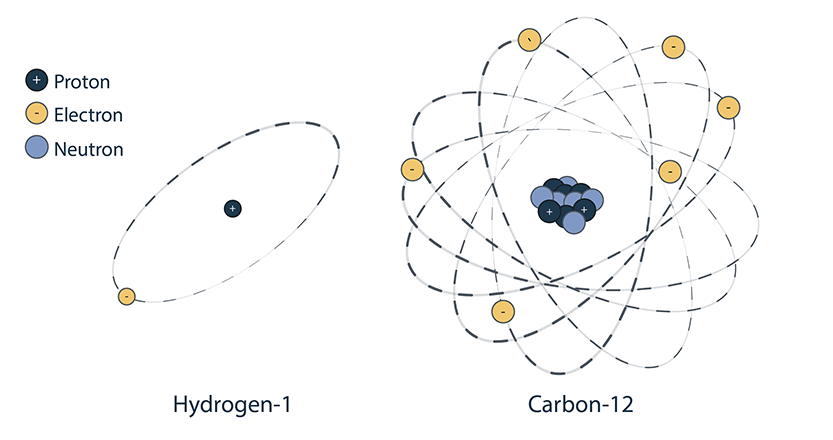
A nuclide is a specific type of atom defined by the total number of protons and neutrons in its nucleus. This number represents the nuclide’s approximate mass and is called the mass number.
A nuclide’s mass number is measured in atomic mass units (amu). For example, Carbon-12 has 6 protons and 6 neutrons, and a mass of 12 amu.

Isotopes
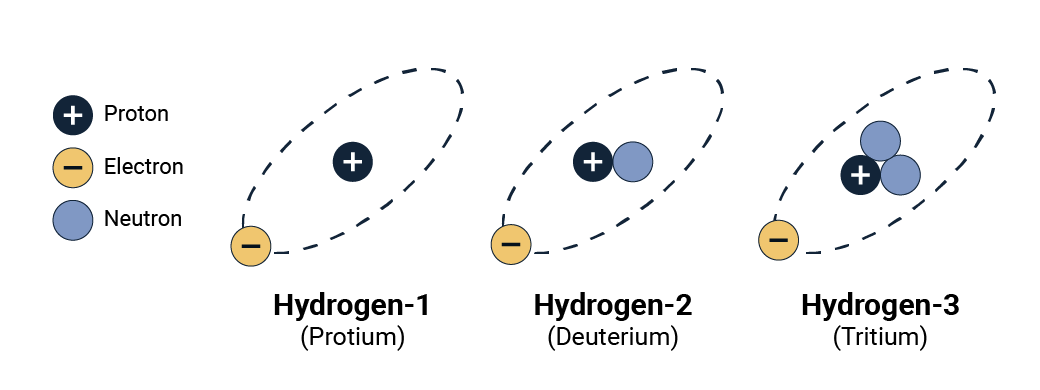
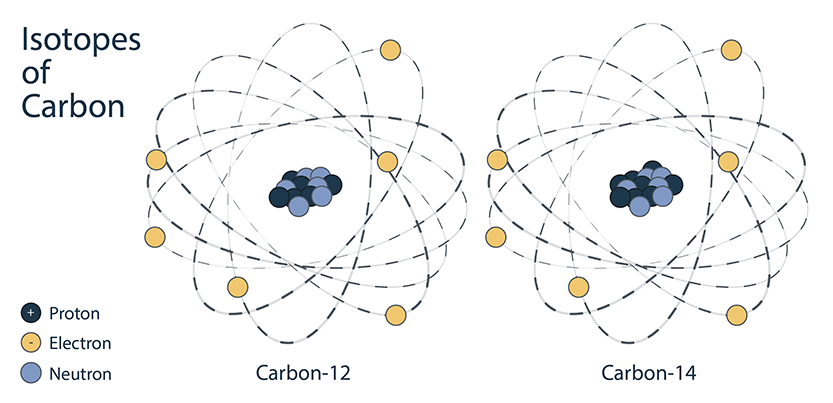
Nuclides of an element that have the same number of protons but a different number of neutrons are called isotopes of that element. They are a variant of a basic element. For example, there are 3 isotopes (or versions) of hydrogen:
- Hydrogen-1 or protium has 1 proton and no neutrons.
- Hydrogen-2 or deuterium has 1 proton and 1 neutron.
- Hydrogen-3 or tritium has 1 proton and 2 neutrons.
Isotopes of hydrogen
Isotopes of uranium include:
- uranium-235, with 92 protons and 143 neutrons
- uranium-238, with 92 protons and 146 neutrons
Stable isotopes
Many isotopes are stable. They do not undergo radioactive decay and do not give off radiation. An isotope is stable when there is a balance between the number of neutrons and protons. This balance does not always mean the 2 are present in equal numbers however.
When an isotope is small and stable, it contains close to an equal number of protons and neutrons. Isotopes that are larger and stable have increasingly more neutrons than protons. This balances repulsion from the positively charged protons near each other.
An example of a stable isotope is carbon-12, which has 6 protons and 6 neutrons, for a total mass of 12 amu.
Unstable isotopes
An unstable isotope is an atom with an unstable nucleus due to an imbalance between protons and neutrons. Unstable isotopes are radioactive, so they are called radioisotopes.
The imbalance often happens when the ratio of neutrons to protons is too low. When this happens, the atom loses energy by giving off ionizing radiation in order to obtain a stable state. This causes the atom to decrease its mass and become a different element. Some atoms may also gain stability through other means, such as spontaneous nuclear fission.
These spontaneous processes that emit energy are known as radioactive decay. Energy can be emitted in the form of alpha particles, beta particles (electrons and positrons) and/or gamma rays. There are 3 main types of radioactive decay:
- alpha decay
- beta decay
- gamma decay
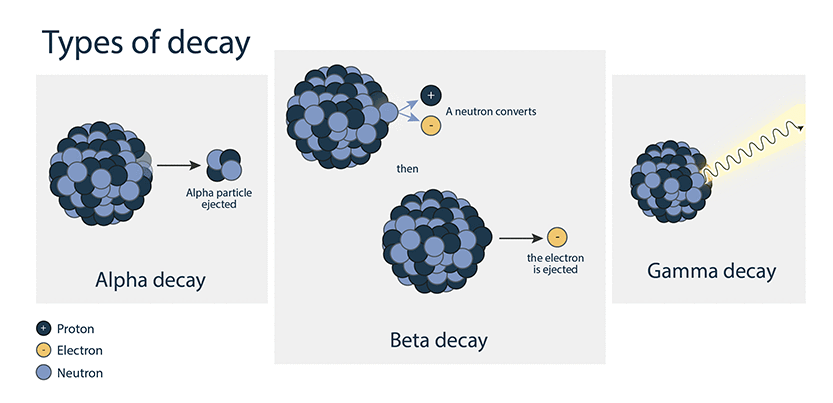
Alpha decay
Alpha decay happens when a particle consisting of 2 neutrons and 2 protons is ejected from an atom’s nucleus.
This causes the atomic number to decrease by 2 and the mass to decrease by 4.
Beta decay
In the most common type of beta decay, a neutron turns into a proton by emitting an electron from the atom’s nucleus.
The atomic number (number of protons) increases by 1. The mass decreases only slightly – because electrons contribute a very small amount to an atom’s mass.
Gamma decay
Gamma decay takes place when there is residual energy in the nucleus following either alpha or beta decay. The residual energy is released as a photon of gamma radiation. Gamma decay generally does not affect the mass or atomic number of the nuclide.
It can also take place after neutron capture in a nuclear reactor.
Radionuclides
When a nuclide disintegrates spontaneously, it emits excess energy that is a form of ionizing radiation called T, in other words, activity. The nuclide that changes and emits radiation is called a radionuclide.
These disintegrations are expressed or measured in a unit called a becquerel (Bq). 1 Bq = 1 disintegration per second.
Half-life
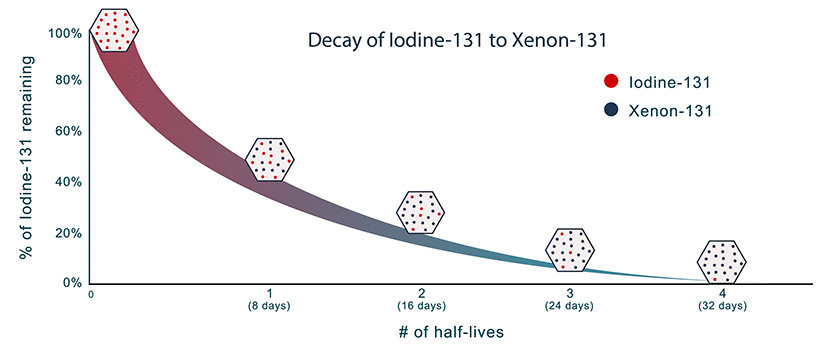
Half-life is the time it takes for a radionuclide to decay to half of its starting activity. The decay is exponential.
Each radionuclide has a unique half-life that can range from a fraction of a second to billions of years. For example, iodine-131 takes 8 days to reach half of its original activity, while plutonium-239 takes 24,000 years.
The symbol for half-life is t½. You can predict how long a radionuclide will take to decay if the original source of the radioactivity is known. The reverse is also true: if you know a radionuclide’s half-life, you can identify the radionuclide.
Specific activity
Specific activity is the activity (the disintegration rate of a nuclide that gives off radiation, or the number of decays per second) per unit of mass of a radionuclide.
Half-life and specific activity have an inversely proportional relationship. The shorter a radionuclide’s half-life is, the more activity the radionuclide gives off.
Cobalt-60, for example, has a shorter half-life than uranium-238 and gives off more radiation per second by gram. It decays more quickly than uranium-238, which gives off less radiation per second by gram and decays more slowly.
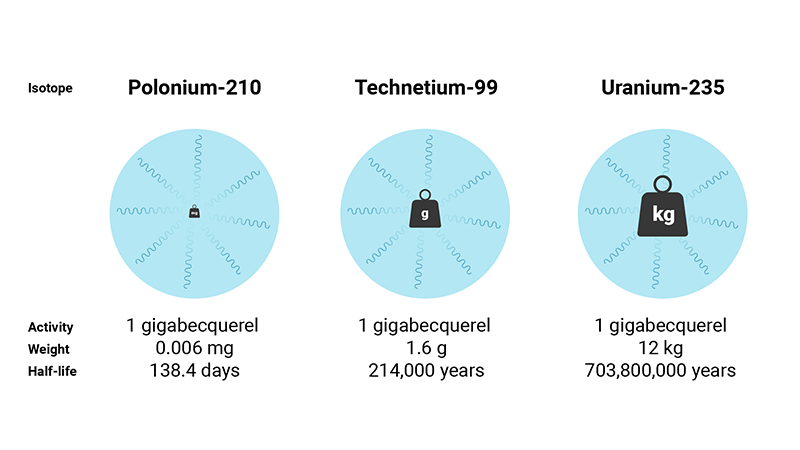
The following table compares different radionuclides with different half-lives and shows the inverse relationship between half-life and specific activity. The last column shows how many milligrams of the isotope are required to yield 1 MBq of activity. Specific activity is expressed in units of becquerels per gram, and depends on each radionuclide’s half-life and atomic mass.
Comparison of radionuclide half-lives and specific activity
| Radionuclide | Half-life | Specific activity* | Milligrams (mg) per megabecquerel (MBq) |
|---|---|---|---|
| Polonium-210 | 138.4 days |
166.3E12 (166.3 TBq/gr) |
0.000006 |
| Cobalt-60 | 5.3 years |
41.9E12 (41.9 TBq/gr) |
0.0000239 |
| Cesium-137 | 30.04 years |
3.2E12 (3.2 TBq/gr) |
0.0003109 |
| Carbon-14 | 5,700 years |
165.7E9 (165.7 GBq/gr) |
0.006 |
| Technetium-99 | 214,000 years |
624.9E6 (624.9 MBq/gr) |
1.6 |
| Uranium-235 | 703.8 million years |
80.0E3 (80.0 kBq/gr) |
12,000.5 |
| Uranium-238 | 4.468 billion years |
12.4E3 (12.4 kBq/gr) |
80,000.4 |
*TBq: terabecquerel (1 trillion Bq)
GBq: gigabecquerel (1 billion Bq)
MBq: megabecquerel (1 million Bq)
kBq: kilobecquerel (1,000 Bq)
Natural versus artificial sources of radionuclides
Many radionuclides occur naturally and originate from the formation of the solar system. Some radionuclides, such as tritium, come from the interaction of cosmic rays with molecules in the atmosphere.
Some radionuclides – like uranium and thorium – that were formed at the time of the solar system’s origin have half-lives of billions of years. They are still in our environment.
Radionuclides are also created as a by-product of the operation of nuclear reactors and by radionuclide generators, such as cyclotrons. Many artificial radionuclides are used in:
- nuclear medicine and biochemistry
- the manufacturing industry
- agriculture
Related links
- Introduction to radiation: main topics
- What is radiation?
- The basics of ionizing radiation
- Nuclear safety
- Radioactive sources safely used
- Nuclear Safety and Control Act (Justice Laws Website)
- Radiation, People and the Environment (International Atomic Energy Agency)
Page details
- Date modified: