Steam-Oxidation of CANDU Reactors Fuel Channels, Feeders and Headers Steel Components
Abstract of the technical paper/presentation presented at:
28th International Conference on Structural Mechanics in Reactor Technology
10 to 15 August 2025
Prepared by:
M. Shawkat and S. Gyepi-Garbrah
Canadian Nuclear Safety Commission
Abstract:
Following a severe accident at a CANDU Nuclear Power Plant (NPP), such as a total station blackout, the fuel and fuel channel integrity will be challenged due to a lack of coolable geometry that results in a mismatch between the rate of heat generation and the heat removal capacity The steam-zircaloy and steam-steel interaction at elevated temperatures will generate hydrogen concentrations that may represent an explosion risk and challenge the containment integrity. Within the CANDU reactor (Figure 1), zircaloy is present in the fuel sheaths, pressure tubes, and calandria tubes while steel is present in the end fittings, liner tubes, channel-closure plugs, shield plugs, feeders and headers.
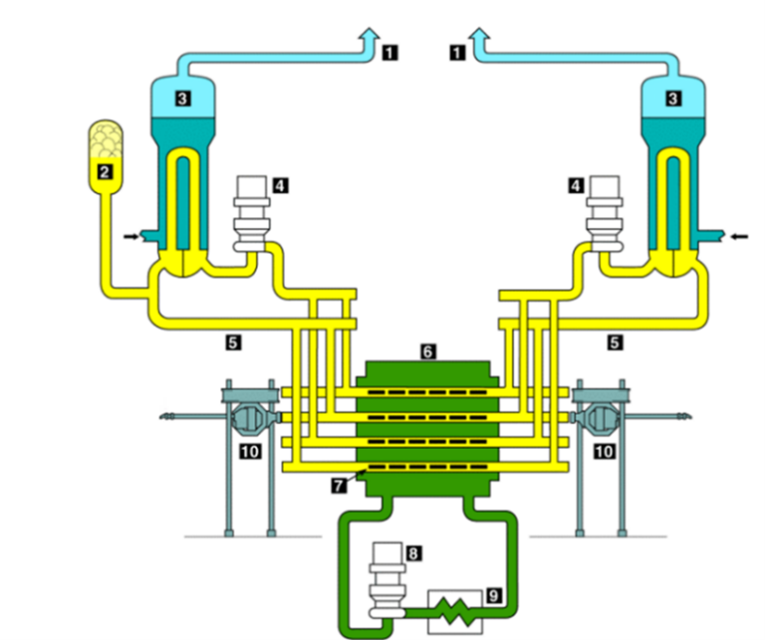
1. Steam line leading to steam turbines 2. Pressurizer 3. Steam generator 4. Pumps 5. Inlet & Outlet headers 6. Calandria vessel 7. Fuel channel 8. Moderator recirculation pump 9. Heat exchanger and 10. Online refueling machines
Figure 1: Schematic of a CANDU reactor illustrating various components and structures
The Canadian NPPs have implemented Severe Accident Management (SAM) programs to identify and mitigate the consequences of severe accidents, including hydrogen combustion risk prevention and mitigation. A SAM program employs several strategies to mitigate the risk of hydrogen by reducing the hydrogen source term using igniters (if power is available), Passive Autocatalytic Recombines (PARs) and dilution techniques via the injection of inert gas into the containment and creating a lean mixture of hydrogen and air below the concentration limits required for the prevention of over pressure challenges to the containment.
Typically, more attention was given to quantifying the hydrogen releases due to steam-zircaloy reaction because of the high temperatures and the large total surface area of the fuel elements. However, since CANDU reactor design involves the use of multiple fuel channels that are connected by several steel components such as end fittings, extended feeder pipes, and headers, steam oxidation of these parts will also provide a source of hydrogen that needs to be investigated and quantified to ensure that the available SAM prevention and mitigation strategies still have sufficient capacity to perform their intended function of reducing or preventing the combustion risk.
The Canadian Nuclear Safety Commission (CNSC) contracted Westinghouse Canada to perform an experimental study to assess the kinetics of steam-steel oxidation and quantify the hydrogen release for seven alloys that represent different components of CANDU fuel channels and their feeder pipes. Each alloy was subjected to several isothermal oxidation tests in a steam environment that covered a temperature range from 900 to 1600 K. Table 1 summarizes the alloys used in the study and their CANDU representative parts. Seventy-six tests were performed with a few tests repeated to quantify the test reproducibility.
| No. | Material | CANDU Heat Transport Part |
|---|---|---|
| 1 | ASME SA-106 Grade C | Feeders Piping in some refurbished units |
| 2 | ASME SA-106 Grade B | Feeders Piping and Header |
| 3 | ASME SA-182 F6a | Channel Closure |
| 4 | ASTM-A564-Grade 630-H1075 | Channel Closure and Shield Plugs in some NPPs |
| 5 | AISI Type 403-SS | End Fitting |
| 6 | ASTM-A268 grade TP-410 SS | Liner tube |
| 7 | ASTM A-395 Grade 60-40-18 (low cobalt) | Shield Plug |
Two approaches were used to quantify the hydrogen release transients: (a) Thermogravimetric Apparatus (TGA) for experiments at 900 K and (b) Ultra-High Temperature (UHT) apparatus for temperatures higher than 900 K. The TGA measures the increase of specimens’ weight during oxidation and which is then used to estimate the hydrogen release assuming stoichiometric oxidation process. In contrast, the UHT can measure the hydrogen release directly, by passing the products of the steam-steel reaction on a chromatography system to detect the hydrogen generation transients without making any assumptions regarding the oxidation process. At 900 K, the initial UHT tests resulted in small hydrogen release that could not be detected with reasonable confidence; therefore, TGA was used at this temperature. The weight gains of the specimen tested in UHT were also measured and compared to the values derived from the measured hydrogen releases to compare the results of the two approaches and quantify the accuracy of assuming the oxide metallurgical phase. As several oxide structures could be formed, X-ray diffraction analysis was performed to selected oxidized specimens to quantify the metallurgical phases of the oxide.
Hydrogen production rates were obtained for all tests at the targeted temperatures except for the melted specimen of A-395 at 1500 K. At the low temperature of 900 K, the increase in chromium content resulted in less oxidation rate. However, at higher temperatures the dependency of the oxidation rate on the chrome content was observed to be less. All hydrogen transient data showed that the steam-steel oxidation kinetics of the tested alloy followed parabolic kinetics and an Arrhenius-type function was used to model the reaction rate constant for each alloy. The x-ray diffraction analysis showed that the formed oxide crystal structures were mainly Magnetite (Fe3O4) and Wuestite (FeO), and the average oxidation state of iron was about a valence of +2.5. The results of this study were in good agreement with the SA-106 Grade B TGA study performed by Canadian Nuclear Laboratories. The weight gain rate constants for the investigated alloys were compared to the zircaloy-steam oxidation values derived based on Cathcart-Pawel (C-P) and Urbanic-Heidrick (U-H) correlations and the results are shown in Figure 2. For the same temperature, the hydrogen generation from zircaloy oxidation is less than the hydrogen generation from the oxidation of most of the alloys investigated. During severe accidents, the temperature of the zircaloy fuel sheaths is expected to be much higher than the rest of the fuel channel components and the feeders since they are in direct contact with the fuel pellets.
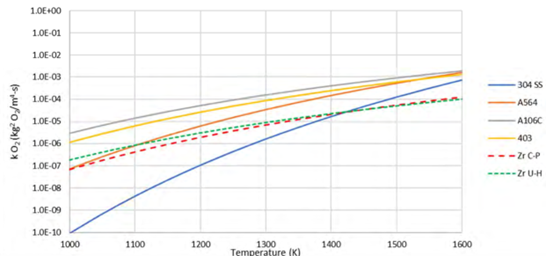
The results of this study demonstrate the importance of predicting the temperature transients for fuel sheath, fuel channel internal steel components, and feeders during severe accident progression using appropriate simulation tools that model the oxidation kinetics of these parts to accurately quantify the hydrogen release rates. This study will help CNSC staff to further develop the scientific capacity and in-depth understanding to oversee the licensee’s use of SAM mitigating strategies for the prevention and mitigation of the hydrogen combustion risk during severe accidents in CANDU reactors.
To obtain a copy of the abstract’s document, please contact us at cnsc.info.ccsn@cnsc-ccsn.gc.ca or call 613-995-5894 or 1-800-668-5284 (in Canada). When contacting us, please provide the title and date of the abstract.
Page details
- Date modified: