Understanding Radiation
Fact Sheet – Understanding Radiation (PDF, 2 pages, 522 KB)
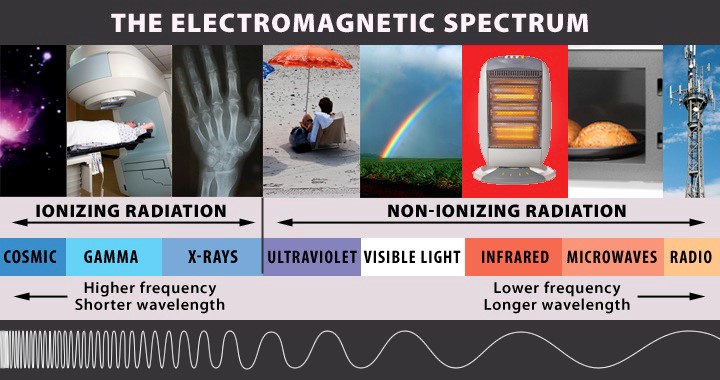
Simply put, radiation is energy in the form of moving waves or streams of particles. This energy can be high-frequency, like X-rays or cosmic rays from outer space or low-frequency, like microwaves and radio waves. This range of energy makes up the electromagnetic spectrum and is divided into two main types of radiation: ionizing and non-ionizing.
Non-ionizing radiation does not possess enough energy to create charged atoms or molecules, called ions. Ionizing radiation, on the other hand, does possess sufficient energy to create ions. Ions can be harmful to your body, but they can also be used for many beneficial purposes.
Quick facts
Ionizing radiation is higher-energy radiation that is capable of removing electrons from atoms as it passes through matter (such as air, water or living tissue). Examples include alpha particles, gamma rays, X-rays and neutrons.
Non-ionizing radiation is radiation of lower energy than ionizing radiation; it does not possess enough energy to produce ions. Examples include visible light, infrared, and radio waves.
Text version
The electromagnetic spectrum is the range of frequencies of electromagnetic radiation. It is divided into two main types of radiation: ionizing and non-ionizing. Ionizing radiation is higher frequency with shorter wavelengths. Examples of ionizing radiation include cosmic rays, gamma rays and x-rays. Non-ionizing radiation is lower frequency and longer wavelengths. Examples of non-ionizing radiation include visible light, infrared and radio waves.
Sources of radiation
We live on a planet where we are exposed to natural background radiation. Radioactive materials are present in soil, rocks, the air we breathe, the water we drink, and even in our own bodies. These sources of natural radiation make up the bulk of the total radiation we are exposed to every day. We are also exposed to artificial radiation from various sources, such as nuclear medicine – which uses radioactive material to diagnose and treat cancer, the nuclear fuel cycle, as well as commercial products like smoke detectors.
Putting radiation doses into perspective
The health effects of radiation are well understood. Since the early 20th century, radiation’s effects have been studied in depth, in both the laboratory and among human populations. Because of the known health risks of radiation, it must be carefully used and strictly controlled. A balance must be struck between radiation’s societal benefits and the risks that radiation poses to people and the environment.
Generally speaking, a dose is the quantity we use when we talk about the potential health effects of radiation. An effective dose takes into account the type of radiation you’ve been exposed to and the organs in your body which have been exposed. It is expressed using the unit "sievert" or more commonly "millisievert" (mSv), which is 1,000 times smaller.
The CNSC sets radiation dose limits for nuclear energy workers and the public in Canada in order to keep radiation doses well below harmful levels to protect human health and safety.
Text version
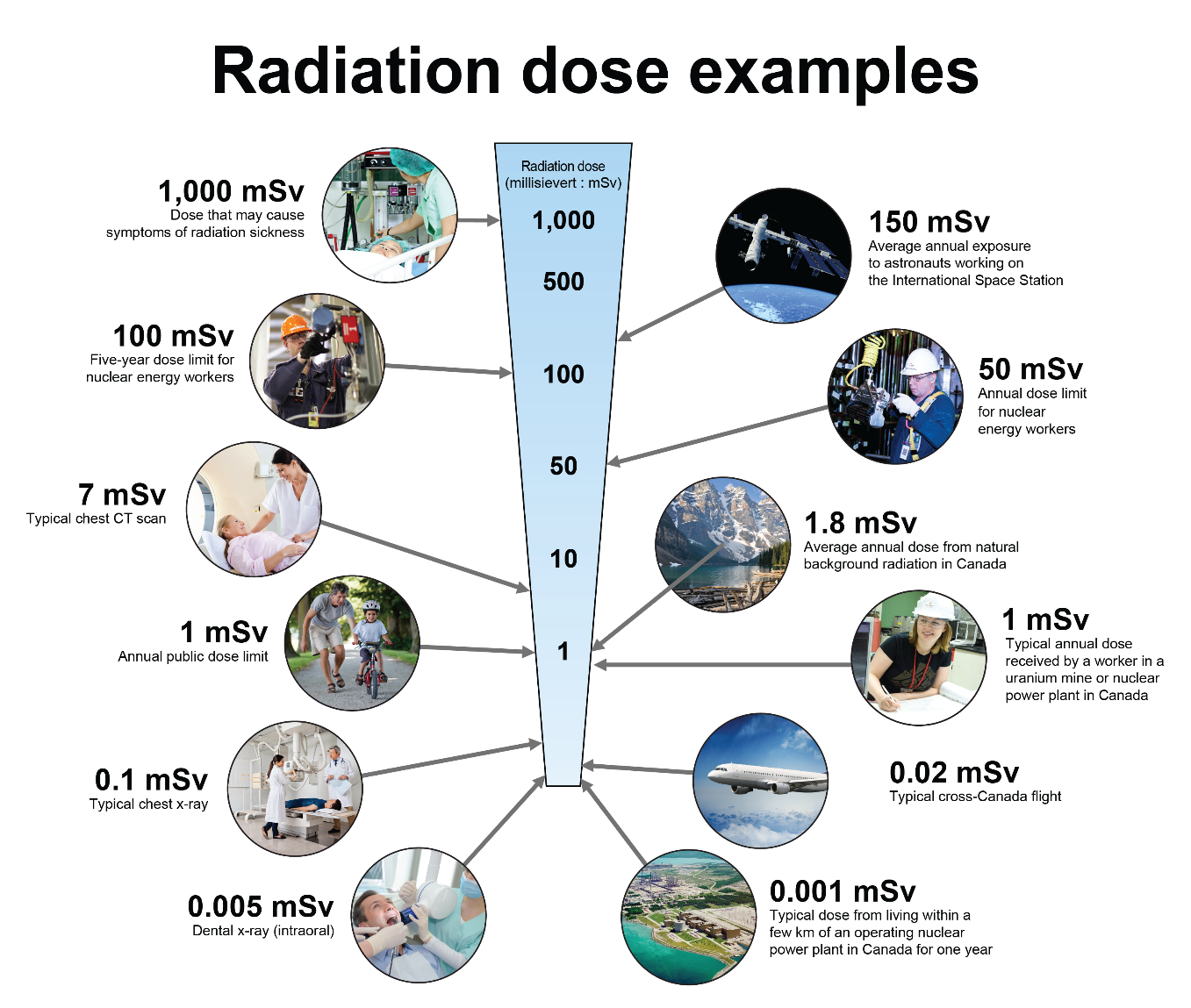
1,000 millisievert dose: May cause symptoms of radiation sickness.
150 millisievert dose: Average annual exposure to astronauts working on the International Space Station.
100 millisievert dose: Five-year dose limit for nuclear energy workers.
50 millisievert dose: Annual dose limit for nuclear energy workers.
7 millisievert dose: Typical chest CT scan.
1.8 millisievert dose: Average annual dose from natural background radiation in Canada.
1 millisievert dose: Annual public dose limit.
1 millisievert dose: Typical annual dose received by a worker in a uranium mine or nuclear power plant in Canada.
0.1 millisievert dose: Typical chest x-ray.
0.02 millisievert dose: Typical cross-Canada flight.
0.005 millisievert dose: Dental x-ray (intraoral).
0.001 millisievert dose: Typical dose from living a few km of an operating nuclear power plant in Canada for one year.
Page details
- Date modified: